Get Free Consultation
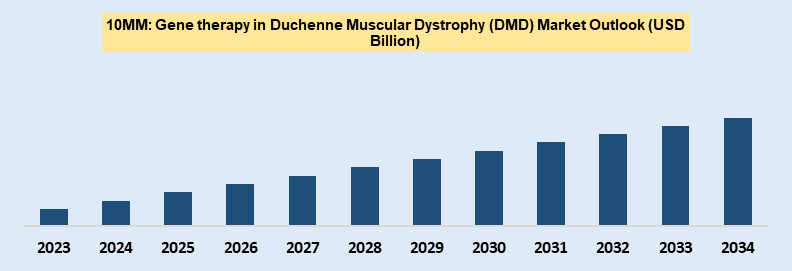
Total Gene therapy Market size was estimated USD 9.21 billion in the 2023, growing at a CAGR of ~xx% during the forecast period. Gene therapy market in DMD indication expected to offer significant $ opportunity USD XX million value over the forecast period.
Gene therapy is a novel way of treatment in DMD, this helps in inducing production of a shortened but functional version of dystrophin, known as a micro-dystrophin protein. Gene therapy is giving new ray hope to affected DMD patients and envisage to revolutionized the treatment landscape.
DMD caused by mutations in a gene for a muscle protein know as dystrophin. The clear role of dystrophin plays in the muscle is still in examination phase, but its loss causes muscle cells to die. Their demise leads to progressive muscle loss. DMD affects nearly 1 in 5,000 males though females are rarely affected. In United States it is estimated that 12,000 people in the U.S. suffer from DMD. In European country such in France annually 150 to 200 young boys are diagnosed with Duchenne muscular dystrophy.
FDA approved drug "Elevidys", a became, first gene therapy product for the treatment of pediatric patients 4 through 5 years of age with Duchenne muscular dystrophy

Devil Hide in the Details: Gene therapy is a very promising way of treatment, but need lot of treatment understanding. Researchers have also pointed out to devise a system to carry the full dystrophin gene as separate pieces in different AAV vectors which could then assemble, Voltron-style, inside the muscle cell. Others are exploring different types of vectors, such as antibodies and tiny fat droplets.
Currently, there is no cure for DMD patients, while there are therapies that can slow down disease progression and improve treatment outcome
Recent Developments in the Market:
Competitive Landscape: (Report section covers both pipeline as well as marketed players)
| Nippon Shinyaku |
| Daiichi Sankyo |
| Dyne Therapeutics |
| PepGen |
| Entrada Therapeutics |
| BioMarin Pharmaceutical |
| Avidity Biosciences |
| Sarepta Therapeutics |
| LambdaGen Therapeutics |
| AAVogen |
| Code Biotherapeutics |
| GenAssist Therapeutics |
| Pfizer |
| GENETHON |
| REGENXBIO |
| HuidaGene Therapeutics Co., Ltd. |
| FibroGen |
| Summit Therapeutics |
| Solid Biosciences Inc. |
| Others |
Reason to buy this report: